Iranians seek medical asylum in Europe as healthcare system collapses
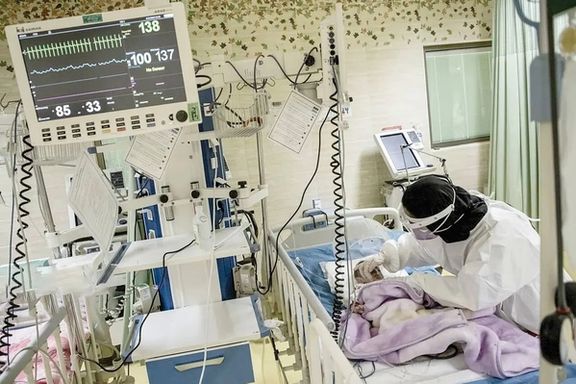
Due to medication shortages in Iran, families of patients with Spinal Muscular Atrophy (SMA) are seeking asylum in Europe amid Iran’s collapsing healthcare system.

Due to medication shortages in Iran, families of patients with Spinal Muscular Atrophy (SMA) are seeking asylum in Europe amid Iran’s collapsing healthcare system.
According to the Ham-Mihan newspaper, patients are seeking refuge in countries like Austria, Germany, the Netherlands, and the UK.
As death rates among SMA patients have also risen sharply, with over 50 people having died since last year, the crisis is worsening in Iran where a flood of medical professionals have fled to seek work abroad.
Saeed Azamian, former director of the SMA Association, said the situation worsened after medication for children under 11 was halted last December, leading to the deaths of at least 20 children suffering from the genetic neuromuscular disease.
In March, Iran International reported that 10 children had died in one month alone as a result of the crisis.
A report published last year also noted that of the 781 patients registered in Iran by October 2022, 164 patients died, the majority of them during the first 20 months of life.
Over the past three years, patients and their families have protested against the Ministry of Health for failing to import or distribute the medication.
Families are selling their homes and belongings to pay brokers and immigration lawyers to seek asylum in European countries. The report cited the case of a woman named only as Zahra, who fled to the Netherlands with her sick six-year-old son. They now live in a camp but receive the expensive medication he needs for free.
Families had previously relied on campaigns for medication, but donations dwindled after President Ebrahim Raisi promised the government would supply the necessary drugs, a promise he failed to keep.
Months passed before the partial import of medicines began and patients had been paying up to 250 million tomans per bottle of Risdiplam syrup, a costly treatment for SMA. The medication was not part of Iran's official drug list until late 2021.
The Ministry of Health claimed it would assess the drug's effectiveness, but results were delayed while the efficacy of drugs like Spinraza and Risdiplam has already been proven in multiple other countries.

Iranian officials have been promoting last year's $5 billion in foreign investment as a success despite sanctions. However, this amount pales in comparison to the much larger sums being attracted by neighboring countries.
The Chairman of the Iranian and Foreign Joint Investment Association, Hossein Salimi, in an interview with ILNA admitted that Iran's energy crisis could be the final nail in the coffin for its already anemic foreign investment prospects. According to Salimi, the ongoing shortfall in energy production and consumption is so severe that it may deter foreign investors more than any other issue.
$5 Billion: A contrast to regional success stories
Iran’s much-publicized $5 billion in foreign investment last year is dwarfed by the figures coming out of neighboring countries. For example, the UAE attracted $22.5 billion in foreign direct investment (FDI) in 2022, and Saudi Arabia, riding the wave of Vision 2030, secured around $21 billion the same year. These numbers are several times higher than Iran’s, highlighting how far the Islamic Republic has fallen behind its regional rivals in the race to attract global capital.
While Iranian officials like Salimi say that the $5 billion investment is a small victory, the reality is that it’s hardly enough to compensate for the country's systemic mismanagement, outdated infrastructure, and the suffocating impact of international sanctions. In comparison, the UAE and Saudi Arabia, free from such constraints, have built an investor-friendly environment supported by stable energy supplies, global trade relationships, and consistent economic reforms.
Energy crisis: A ticking time bomb
Salimi’s comments underscore a critical point: even if foreign companies wanted to invest in Iran, they would face challenges due to the country’s dysfunctional energy sector. Years of underinvestment, combined with poor management, have left Iran with a 14,000-megawatt electricity deficit. As a result, the country is struggling to meet even its current consumption, let alone the additional energy needs of foreign industrial investors.

Salimi’s warning points to a reality: "If tens or hundreds of foreign industrial companies come to Iran... will we be able to provide the necessary water, electricity, and gas for these industries?" The answer, it seems, is no. Any influx of foreign business would likely run into the same problems that Iranian citizens already face—frequent blackouts, water shortages, and limited access to energy resources.
The problems run deeper than just energy shortages. Salimi’s reminder of energy giant Total's departure from Iran is emblematic of how the country’s geopolitical entanglements have stunted its growth. In 2018, after the US reimposed sanctions, Total, one of the world's largest oil and gas companies, pulled out of a multibillion-dollar gas deal, signaling to the world that Iran was simply too risky for long-term investment. Since then, the situation has only worsened, with Iran remaining on the Financial Action Task Force (FATF) blacklist, limiting its ability to engage in international banking and financial exchanges.
Small investments, big problems
Salimi's mention of the $5 billion in foreign investment last year underscores another issue: the size and type of investments Iran is attracting. According to Salimi, 10-20% of this investment came from Iranians living abroad, while small Afghan investors accounted for less than 20%. More than half of the remaining investment came from companies outside these two groups, mostly in industries like food production, which require relatively small capital outlays.
Meanwhile, an energy expert, Morteza Behroozifar, warns that Iran’s oil industry alone needs $250 to $300 billion in investment to remain afloat. “We are currently experiencing shortages of gas, gasoline, and diesel," Behroozifar said in a recent interview with ISNA, adding that without massive foreign investment and a change in management, Iran’s energy sector is headed for collapse. The $5 billion that officials are highlighting is a fraction of what is required to stabilize and grow the economy.
The road ahead: Will Pezeshkian's government deliver?
President Masoud Pezeshkian has acknowledged the need for at least $100 billion in foreign investment to tackle the country’s growing problems. Yet, according to observers, attracting that kind of capital will require more than just rhetoric—it will demand systemic reform, transparency, and above all, the resolution of Iran’s energy crisis.
With a 14,000-megawatt electricity deficit, Pezeshkian’s cabinet will have to overhaul the country’s infrastructure while navigating the complex web of sanctions and international isolation that has crippled previous administrations.

Despite a change in government, Iran's private sector remains under pressure as the judiciary sentenced the former head of the Chamber of Commerce to prison and a fine.
Iran's Chamber of Commerce, Industries, Mines, and Agriculture (ICCIMA) serves as the voice and the representative body for the private sector. However, the government maintains a tight grip on the chamber, often resisting its efforts to operate independently.
After about a year of controversy surrounding Hossein Selahvarzi’s election as head of the chamber, which eventually led to his dismissal late last year, *Mizan News Agency*, linked to Iran's judiciary, reported on Sunday that the Tehran Court of Appeals sentenced him to six months in prison and a fine for "spreading lies." The campaign against Selahvarzi was reportedly orchestrated by figures close to former President Raisi's administration, further intensifying the political nature of his case.
Last year, EcoIran reported that Tehran deputy prosecutor general had issued an order for the indictment and prosecution of Selahvarzi on charges of insulting the founder of the Islamic Republic Ruhollah Khomeini and Supreme Leader Ali Khamenei in the Tehran Revolutionary Court.
He has frequently criticized officials and their policies in his tweets, and it appears that the main reason for this verdict is his tweets criticizing authorities and supporting protesters during the 2022 protests.

Known for his criticism of government’s economic policies, Selahvarzi was elected as the president of the Chamber of Commerce in June 2023. The election immediately sparked reactions from the hardliners and state media. A few days after Selahvarzi’s election, around 30 hardliner lawmakers had urged the government to annul the elections.
IRGC-affiliated Tasnim News Agency published images of his social media posts from the 2022 nationwide protests for "Woman, Life, Freedom," labeling the Chamber of Commerce president as a "dissident. Mehr News Agency also released documents claiming that Selahvarzi had been disqualified by the Ministry of Intelligence before the Chamber of Commerce board election.
On several occasions, the state media announced that Selahvarzi had resigned but he denied the reports himself on social media. Finally in November, Selahvarzi was dismissed by the Minister of Economy.
Over the years, Selahvarzi has held various economic positions, though he is politically aligned with the so-called Reformists. While the election of reform-minded President Masoud Pezeshkian raised hopes for a shift in economic policy, the entrenched influence of the previous administration's hardline economic stance seems to have limited the potential for significant change.
The chairman of the chamber, often referred to as the “private sector’s parliament” with over 400 members, has the role of coordinating between the private sector and government and is sometimes required to participate in meetings with government officials.
Members of the chamber have often criticized regime policies that have led to an economic crisis, including a confrontational foreign policy. The chambers often produce economic reports that the government finds embarrassing, or they criticize proposed budget bills and other plans.
“Chairmanship of the chamber of commerce is not a government position in the Islamic Republic. The chamber is a non-governmental and non-state body and represents the private sector,” Abdolreza Davari, one of former President Mahmoud Ahmadinejad's advisors and confidants, said last year, calling “politically-motivated interference” in chamber of commerce elections by the state “a dangerous phenomenon”. “The affairs of the chamber of commerce should be decided and regulated by the members of the chamber itself,” he added.

Ukraine's foreign ministry summoned Iran’s charge d’affaires Monday to warn Tehran against the consequences of ballistic missile shipments to Russia.
A report published last week by The Wall Street Journal suggested that Iran had transferred short-range ballistic missiles to Russia, prompting outrage in Kyiv. At least one Iranian lawmaker confirmed the report Sunday, conflicting a senior Revolutionary Guards commander who had dismissed the claim as “psychological warfare.”
White House national security spokesperson John Kirby added a layer of uncertainty to the situation Monday, when he refused to verify the Wall Street Journal report.
“I cannot confirm the reports that the transfer has happened,” he said in a presser, “but I would point you to what we've said in the past, that any such provision of that kind of technology… could, depending on what the deal, how it's consummated, have equally deleterious effects on the Middle East.”
“An Iran that already has an improving ballistic missile program, we can only assume would want to stand to gain from some sort of partnership with Russia to improve their capabilities in the region,” Kirby added.
Iran is known to have sent Russia drones, but a provision of ballistic missiles would be seen as a serious escalation. So far, no such deal has been officially announced. "No missile was sent to Russia," Brigadier Fazlollah Nozari, a high-ranking IRGC commander said Monday, calling the allegations "psychological warfare".
'Devastating consequences'
On Monday, Kyiv warned Iran that confirmation of such missile deliveries would have "devastating and irreparable consequences" for bilateral relations. Senior Ukrainian official Andrii Yermak further called for Western support, suggesting that his country “must be allowed to destroy warehouses storing these missiles in order to avoid terror."
Iran’s growing support for Russia’s war effort has also been criticized in Tehran. On Sunday, a former Iranian lawmaker who used to chair of the committee on national security and foreign policy slammed Iran's arms sales to Russia as "the dirtiest example of Russophilia", warning that the leaders of the Islamic Republic are “dragging” the country “further into global conflict and sanctions which have led to the country's worst economic crisis” in more than four decades.
Also on Sunday, a sitting Iranian MP seemed to confirm Tehran’s military assistance to Russia, albeit downplaying the threats from Ukraine and its Western allies. Ahmad Bakhshayesh Ardestani justified the arms sales to Russia by saying his country has to subsequently "barter" for its needs, including soybeans and wheat. "Part of the barter involves sending missiles, and another part involves sending military drones to Russia," he said.
When asked whether sending ballistic missiles to Russia might lead to further sanctions or trigger the so-called "snapback" mechanism against Iran, the member of the Parliament's National Security and Foreign Policy Committee replied, "It can't get any worse than it already is. We give missiles to [our proxies] Hezbollah, Hamas, and Hashd al-Shaabi, so why not to Russia?"

Rafael Grossi, the Director General of the International Atomic Energy Agency (IAEA), has raised alarms over the continuous increase in Iran’s stockpile of uranium enriched to 20% and 60%.
Grossi also highlighted the agency's growing concerns over Tehran’s failure to provide critical information about its nuclear program, further escalating international fears of Iran's advancing nuclear capabilities.
"Iran’s stockpile of uranium enriched up to 20% and up to 60% continues to increase, and that Iran has expanded the number of cascades it is using to enrich UF6," said Grossi, in his latest statement on the Iranian nuclear issue.
The diplomatic landscape surrounding Iran’s nuclear program has been tense for years, but recent developments have escalated the situation. Iran halted its implementation of nuclear commitments under the Joint Comprehensive Plan of Action (JCPOA) more than three years ago, and its stockpile of enriched uranium continues to rise. With international inspectors barred from accessing key facilities, the IAEA now finds itself in the dark about Iran’s centrifuge inventory, uranium ore, and heavy water stocks.
"The Agency has lost continuity of knowledge in relation to the production and inventory of centrifuges, rotors and bellows, heavy water and uranium ore concentrate," Grossi explained, underscoring the gaps in oversight created by Tehran’s refusal to cooperate.
Iran's defiance has not been limited to the technical aspects of nuclear verification. Grossi pointed out that Iran has failed to resolve "outstanding safeguards issues," particularly regarding the discovery of uranium particles at undeclared sites, a discovery that contradicts Tehran’s claims of transparency.
As the IAEA calls for renewed cooperation, Iran has instead accelerated its nuclear enrichment efforts. According to the latest reports, the installation of additional centrifuge cascades at the Natanz and Fordow nuclear facilities has intensified, bringing the nation closer to reaching the 90% purity required for a nuclear weapon.
This expansion comes on the heels of repeated IAEA requests for Iran to resume compliance with the JCPOA, an agreement Tehran abandoned following the US withdrawal under the Trump administration. Since then, efforts to reinstate or renegotiate the deal have stalled.
"I call upon Iran to implement the Joint Statement through serious engagement with the Agency’s concrete proposals," Grossi urged, signaling a desire to reestablish dialogue with the newly elected Iranian leadership.
"He (Pezeshkian) agreed to meet with me at an appropriate juncture," Grossi said, referring to an exchange after Pezeshkian's election in July.
"I encourage Iran to facilitate such a meeting in the not-too-distant future so that we can establish a constructive dialogue that leads swiftly to real results," he added.
In July, US Secretary of State Antony Blinken noted that Iran’s nuclear breakout time—the time needed to produce enough 90% enriched uranium for a nuclear weapon—has likely shrunk to "one or two weeks."
Reports indicate that Iran is ramping up efforts in its secretive nuclear weapons program, edging the country closer than ever to the development of a nuclear bomb, a threat that has persisted for over two decades.
Earlier this month, Abbas Araghchi, Iran's newly appointed Foreign Minister, stated that reviving the 2015 JCPOA nuclear agreement with the six major world powers is "untenable in its current form."

An Iranian dissident man was gang-raped in Germany by four men who are believed to be Islamic Republic loyalists, in an attack that has shocked the town of Iserlohn in North Rhine-Westphalia, according to German media reports.
The incident, which occurred on Saturday night at an abandoned brewery site, is being investigated as a politically motivated crime with deep ties to Tehran’s repressive government, according to a report by Der Spiegel.
Witnesses, alarmed by the victim’s screams, alerted the authorities, who responded quickly. IKZ reports that emergency services found the victim tied up and severely abused. He was immediately rushed to a hospital, but the full extent of his injuries has not been disclosed.
The attackers fled the scene, but law enforcement, including a helicopter and multiple patrol units, initiated a rapid search and apprehended four suspects—aged 24, 34, 42, and 46—shortly after in a nearby forest. One of the suspects sustained injuries while attempting to evade capture.
Spiegel and WDR report that all four men are believed to be ardent supporters of the Islamic Republic, underlining the political nature of the attack.
According to information obtained by Spiegel, the 30-year-old victim, an Iranian refugee who had fled persecution back home, was targeted due to his outspoken opposition to the Islamic Republic. The alleged perpetrators—four men with alleged loyalty to the Islamic Republic—sought to "sexually humiliate" and silence him, according to German authorities.
'Sexual humiliation'
"Based on the current state of knowledge, the victim should above all be humiliated," said Michael Burggräf, the prosecutor of Hagen in North Rhine-Westphalia, highlighting the intent behind the attack: to silence dissent through sexual violence. "The attack was primarily intended to sexually humiliate the victim."
The German prosecutor's remarks reinforce the suspicion that the assault was more than a mere political dispute and was rather an orchestrated act of brutality meant to break the spirit of a dissident.
This act of violence is being seen as an extension of the Islamic Republic’s long-standing campaign of intimidation and repression against dissidents, even beyond Iran’s borders.
The clerical government has a well-documented history of targeting its critics and defectors, often using extreme measures to silence opposition. The brutal nature of the latest assault echoes the tactics employed by Iran’s intelligence apparatus, which has been linked to various forms of violence and coercion against dissidents in Europe.
Dozens of assassinations have been attempted ever since the 1979 Revolution, often involving non-Iranians that help those who order the attacks deny complicity.
In June, another Iranian dissident escaped an assassination attempt in the Netherlands in what seemed to be yet another sign of Iran’s resolve to silence voices of dissent outside the country. The targeted dissident, Siamak Tadayon Tahmasebi, was singled out in an Iranian intelligence press release last summer, accused of leading a “terrorist” ring inside Iran from exile. On 6 June, Tahmasebi noticed two men attempting to enter his home near Amsterdam. He alerted the police, who arrived in time to arrest the armed intruders.
In May, Israeli and Swedish intelligence agencies revealed that criminal gangs operating on Iran’s behest were behind several terror attacks on Israeli embassies in Europe.
Over the past few years, Iranian journalists working outside Iran have become a prime target for Tehran's campaign of terror and intimidation. Iran International presenter Pouria Zeraati was stabbed outside his home in south London in March but survived with injuries to his leg. Investigations into the crime suggested that the three suspected assailants were recruited in Eastern Europe and flown to the UK to "intimidate" Zeraati. Also in June, another Iran International reporter, Mehran Abbasian, had to be moved to a secure location following threats to his life.
The threats against Iran International staff have become a recurrent issue, stemming back to 2022. The threats reached a climax with the UK's MI5 saying it could no longer protect the team, forcing a temporary relocation to the US.






